Can-SOLVE CKD Network Annual Meeting 2021 project video
Project lead: Dr. York Pei
Patient lead: David Hillier
Patient group: Adult patients with polycystic kidney disease without end-stage renal disease
Region: All regions
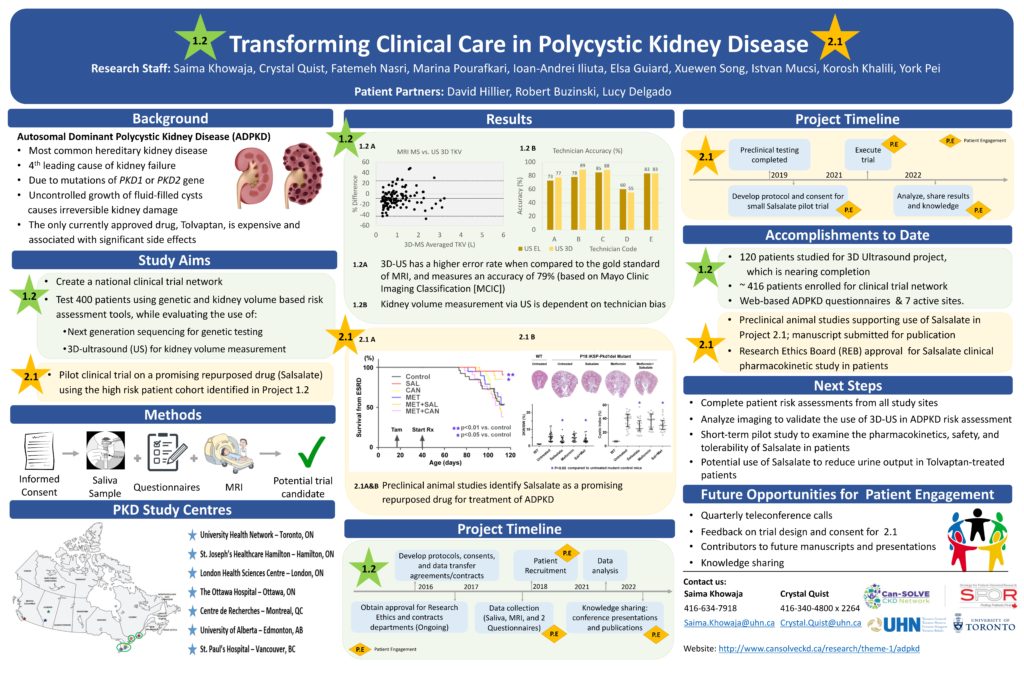
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease. There are two types of ADPKD due to mutations of two different genes: PKD1 and PKD2. PKD1 is generally more severe and accounts for 75% of cases, while PKD 2 accounts for 25%. There is currently no cure for ADPKD; blood pressure control and a healthy lifestyle are the only ways to slow down the disease.
Advances in ADPKD research have identified a number of promising therapeutic drugs. Several of these drugs are re-purposed compounds that are currently approved for diseases other than ADPKD. Although these drugs are inexpensive and safe for long term use, they will not undergo clinical testing to see if they work in patients with ADPKD. Clinical trials are expensive and the companies who make the drugs will not test them due to expiry of the drug patents and the low cost of these medications.
We plan to perform two pilot control trials of two of these therapeutic drugs to test the impact on ADPKD patients and to evaluate their potential. Using the patients identified from Project 1.2 (page 4) and a network of ADPKD research centres from across Canada, we will test the effectiveness and safety of these therapeutic drugs for treating ADPKD. Patient and family input will be included in the design and execution of these studies. This work will transform patient care by providing new treatments to slow disease progression.
Can-SOLVE CKD Network Annual Meeting 2021 visual abstract

Connect with us